You are here
Distributing Promising Covid-19 treatment offers test run for vaccine distribution
Primary tabs
Fri, 2020-08-07 21:25 — mike kraft
 Promising Covid-19 treatment offers test run for vaccine distribution An antibody treatment designed to protect against coronavirus could be available as early as this fall -- but only for a fraction of the millions of Americans who might benefit from the treatment. CNN
Promising Covid-19 treatment offers test run for vaccine distribution An antibody treatment designed to protect against coronavirus could be available as early as this fall -- but only for a fraction of the millions of Americans who might benefit from the treatment. CNN
 Promising Covid-19 treatment offers test run for vaccine distribution An antibody treatment designed to protect against coronavirus could be available as early as this fall -- but only for a fraction of the millions of Americans who might benefit from the treatment. CNN
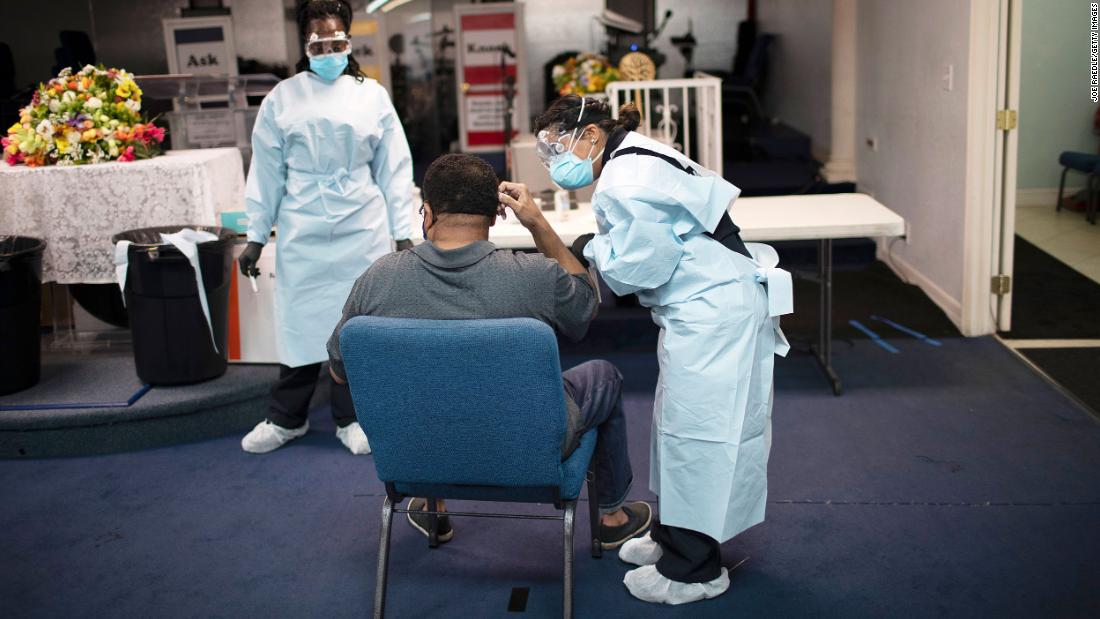
Promising Covid-19 treatment offers test run for vaccine distribution An antibody treatment designed to protect against coronavirus could be available as early as this fall -- but only for a fraction of the millions of Americans who might benefit from the treatment. CNN Washington (CNN)An antibody treatment designed to protect against coronavirus could be available as early as this fall -- but only for a fraction of the millions of Americans who might benefit from the treatment.
Companies are already testing monoclonal antibody therapies -- antibodies created in a lab to target Covid-19 -- in humans. The trials are determining whether the antibodies can prevent people from becoming infected and whether the treatments can stave off the worst symptoms of coronavirus when a patient does become infected.
There's no guarantee they will work.
If they are proven effective, they could be available to Americans months before a vaccine is ready. But according to drug-makers, the initial distribution will most likely be left up to the federal government, whose shoddy track record on setting up widespread testing, distributing personal protective gear and dispensing other drugs such as remdesivir to fight coronavirus is already setting off alarms.
"This will be a test run for allocation of vaccines," said Rep. Bill Foster, an Illinois Democrat who has asked the Government Accountability Office for enhanced oversight, and regular updates, on the therapeutic manufacturing process. "This scramble for monoclonal antibodies could happen two months from now or even earlier."
It's not clear who will make the decisions about where the initial antibody doses go...
It's not clear who will make the decisions about where the initial antibody doses go...
Country / Region Tags:
Problem, Solution, SitRep, or ?:
Groups this Group Post belongs to:
- Private group -



Recent Comments